Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Recent Advances in Actinide Science, p.596 - 598, 2006/06
Recently, extraction selectivity for trivalent minor actinides (MA = Am and Cm) over lanthanides (Ln) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III) [1]. However, protonation of R-BTP results in its acidic hydrolysis in acidic medium. Stability in acidic solution was improved by substitution of long normal chain or branched chain [2]. In this work, separation of MA(III) and Ln(III) from nitric acid solution was studied by using novel R-BTP impregnated resin. Branched R-BTP resin had high affinity for Am from up to 4 M HNO solution and its distribution coefficient was over 10
solution and its distribution coefficient was over 10 .
.
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Alloys and Compounds, 408-412, p.1274 - 1277, 2006/02
Times Cited Count:39 Percentile:84.47(Chemistry, Physical)For the development of advanced aqueous reprocessing system, it is one of the most important subjects to separate minor trivalent actinides (MA = Am and Cm). Recently, extraction selectivity for MA(III) over Ln(III) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III). The novel silica-based extraction resins were prepared by impregnating some R-BTP molecules into a macroreticular styrene-divinylbenzene copolymer which is immobilized in porous silica particles with a mean diameter of 50  m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor (
m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor ( 10
10 ) for Am, and all the elements were recovered quantitatively.
) for Am, and all the elements were recovered quantitatively.
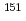 Eu-M
Eu-M ssbauer spectroscopic and X-ray diffraction study of the Eu
ssbauer spectroscopic and X-ray diffraction study of the Eu (Ce
(Ce Zr
Zr )
) O
O and LnEuZr
and LnEuZr O
O (Ln=lanthanide) systems
(Ln=lanthanide) systemsMasaki, Nobuyuki; Nakamura, Akio; Furuuchi, Fumihito*; Hinatsu, Yukio*
Journal of Physics and Chemistry of Solids, 66(2-4), p.312 - 317, 2005/02
Times Cited Count:10 Percentile:42.74(Chemistry, Multidisciplinary)no abstracts in English
Watanabe, Masayuki; Morita, Yasuji; Kubota, Masumitsu*; Tatsugae, Ryozo*
Radiochimica Acta, 87(3-4), p.115 - 119, 1999/00
no abstracts in English
*; Nagasaki, Shinya*; *; Tanaka, Satoru*; Suzuki, Atsuyuki*; Tanaka, Tadao; Muraoka, Susumu
Radiochimica Acta, 82, p.239 - 242, 1998/00
no abstracts in English
Sano, Yuichi; *; ; *; Koyama, Tomozo; Tanaka, Yasumasa
PNC TN8410 96-362, 19 Pages, 1996/10
The coordination properties of the lanthanide (La, Ce, Pr, Nd, Sm and Eu) complexes in lanthanide/TBP (tributylphosphate), lanthanide/CMPO (octyl(phenyl)-N,N-diisobutycarbamoylmethylphosphine oxide) and lanthanide/CMPO/TBP systems were investigated by NMR (nuclear magnetic resonance) measurements. In the lanthanide/CMPO/TBP system, it is shown that the structure of lanthanide complex is changed by the concentration ratio for added CMPO to the lanthanide ion ; lanthanide/CMPO/TBP system ([CMPO]/[Ln]  3 (mole ratio)) NO
3 (mole ratio)) NO - the similar coordination in the Ln/TBP system TBP, CMPO - These coordinate to the lanthanide ion together. (the existence of several complexes) lanthanide/CMPO/TBP system ([CMPO]/[Ln}
- the similar coordination in the Ln/TBP system TBP, CMPO - These coordinate to the lanthanide ion together. (the existence of several complexes) lanthanide/CMPO/TBP system ([CMPO]/[Ln}  3(mole ratio)) NO
3(mole ratio)) NO - the same coordination in the Ln/CMPO system TBP, CMPO - Only CMPO coordinates to the lanthanide ion. TBP doesn't exist within the first coordination sphere, but affects the CMPO exchange reaction.
- the same coordination in the Ln/CMPO system TBP, CMPO - Only CMPO coordinates to the lanthanide ion. TBP doesn't exist within the first coordination sphere, but affects the CMPO exchange reaction.
*; Wakaida, Ikuo
JAERI-Data/Code 96-017, 62 Pages, 1996/03
no abstracts in English
; Aose, Shinichi; ;
PNC TN8410 95-313, 28 Pages, 1995/10
THe coordination properties of the lanthanide (La, Ce, Pr, Nd, Sm and Eu) complexes in lanthanide/TBP (tributylphosphate), lanthanide/CMPO (octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide) and lanthanide/CMPO/TBP systems were investigated by the NMR (nuclear magnetic resonance) measurements. The numbers of the coordinated CMPO and TBP to the lanthanide ion were estimated about three and two in the lanthanide/CMPO and lanthanide/TBP systems, respectively. It is considered that TBP and CMPO coordinate to the lanthanide (III) ion in the monodentate and the bidentate manners, respectively. In the lanthanide/CMPO/TBP system, 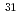 P-NMR spectra suggested that CMPO coordinates to lanthanide(III) ion directly in the bidentate mode, but TBP doesn't exist within the first coordination sphere and coordinates to the lanthanide(III) ion from beyond the first coordination sphere. The activation parameters for the ligand exchange reactions calculated by the CBS (complete bandshape) method suggest that the ligand exchange reactions in the lanthanide (Pr and Eu)/TBP and lanthanide (La, Pr and Sm)/CMPO systems proceed through either the associative (A) mechanism or the dissociative (D) mechanism with an ordering into the second coordination sphere. In the lanthanide (Pr and Sm)/CMPO/TBP systems, it was shown that the CMPO exchange reactions proceed through the mechanism with an ordering into the second coordination sphere, which is caused by TBP in the systems.
P-NMR spectra suggested that CMPO coordinates to lanthanide(III) ion directly in the bidentate mode, but TBP doesn't exist within the first coordination sphere and coordinates to the lanthanide(III) ion from beyond the first coordination sphere. The activation parameters for the ligand exchange reactions calculated by the CBS (complete bandshape) method suggest that the ligand exchange reactions in the lanthanide (Pr and Eu)/TBP and lanthanide (La, Pr and Sm)/CMPO systems proceed through either the associative (A) mechanism or the dissociative (D) mechanism with an ordering into the second coordination sphere. In the lanthanide (Pr and Sm)/CMPO/TBP systems, it was shown that the CMPO exchange reactions proceed through the mechanism with an ordering into the second coordination sphere, which is caused by TBP in the systems.
J.Rais*; Tachimori, Shoichi
Sep. Sci. Technol., 29(10), p.1347 - 1365, 1994/00
Times Cited Count:45 Percentile:89.74(Chemistry, Multidisciplinary)no abstracts in English
 -butyl phosphate system
-butyl phosphate systemJournal of Inorganic and Nuclear Chemistry, 26, p.619 - 625, 1964/00
Times Cited Count:15no abstracts in English